
Alviniconcha hessleri (Mollusca: Mesogastropoda: Provannidae)
When you think of hydrothermal vents, what comes to mind first? Is it the gushing black smoke out of a chimney? Perhaps you envision the enormous tubeworms with their red velvety plumes sticking out of their white tubes. Some may even be familiar with the dense swarms of blind shrimp. What may not come to mind are big hairy snails!
Description Alviniconcha hessleri was discovered and described almost 20 years ago (7) and is a mesogastropod in the family Provannidae (14). It is named both after the submersible Alvin (“Alvin’s shell”) and the preeminent deep-sea biologist, Robert R. Hessler. The snail is roughly spherical in shape and has a thin carbonate shell (if allowed to air-dry, it will fall to pieces within weeks!) The protoconch is almost never visible and juvenile shells show considerable variation in sculpture (3, 14). The spiny appearance of this snail is part of the periostracum and can erode away in larger specimens. Speaking of size, we have seen specimens up to 88mm in length, larger than a softball!

In-situ photographs courtesy of Charles Fisher and photographs of shells on white are courtesy of Linda Zelnio

Physiology A. hessleri has blue blood due to hemocyanin, but also contains hemoglobin in concentrations similar to chemoautotrophic bivalves from other hydrothermal vents (15). The gill of this beast is enlarged and can get up to 21% of the total soft-body mass (dry weight, unpublished data). A normal snail’s gills from the intertidal may weigh around 4-7% of the total soft-body weight.
Nutrition The digestive system is very reduced for the size of this snail. For instance, Warén & Bouchet (14) state, “The stomach is small, about 10mm3 in a specimen 45mm high and a shell volume of about 60,000 mm3. A specimen of Provanna living in the same environment, grazing the surface, has a stomach of 1.5mm3 at a shell of 75mm3. This means that Provanna has a stomach which is 100 times large in relation to the body volume.” (pg. 63) A. hessleri relies on sulfur-oxidizing (9) endosymbiont gamma- or epsilon-proteobacteria for the bulk of its nutrition, which may be supplemented by grazing biofilm off of rocks and digesting the mucus and embedded particles inside the mucus. These bacteria reside inside bacteriocytes (9) in the gills of the snails and provide the snail with the bulk of its nutrition (8, 10),. Interestingly, the occurrence epsilon proteobacteria as chemoautotrophic endosymbionts was a new discovery and occurs only in Alviniconcha from the Central Indian ridge (11) and Manus Basin (12). As Suzuki et al. state, “… at least two lineages of chemoautotrophic bacteria, phylogenetically distinct at the subdivision level, occur as the primary endosymbiont in one host animal type (11).” Epsilon proteobacteria utilize a different carbon metabolism pathway than gamma proteobacteria and hence have different stable isotope ratios (10). Reproduction A. hessleri has separate sexes. They lay down flat, semi-transparent egg capsules on the rock which contains about 20-25 embryos (14). Larvae of A. hessleri have a veliger stage and are presumed to be planktotrophic (14).
Ecology A. hessleri is dominant both numerically and in terms of biomass at the Mariana Trough (3) and shares its dominance with fellow mollusks Ifremeria nautilei and Bathymodiolus brevior at North Fiji and Lau Basins ((2), unpublished data). Several copepods of the genus Stygiopontius colonize the palial cavity and the small polychaete Amphisamytha cf galapagensis sometimes lives in tubes it creates in the groove of the shell whorls. A. hessleri commonly forms the center of a “bull’s eye” pattern we observe at the Lau Basin (documented by Warén & Bouchet (14) for the North Fiji basin), where is occupied the hottest part of the hydrothermal field with the highest concentrations of sulfide (unpublished data). Crabs of the genus Austinograea (both A. alayseae and A. williamsi), the alvinocarid shrimp Chorocaris vandoverae, and the alvinellid polychaete Paralvinella fijiensis (also correlated with high temperatures) have strong associations with beds of A. hessleri (unpublished data). Interestingly, there is a distinct lack of other gastropods, especially limpets, living within the bed of Alviniconcha. My “pet hypothesis” is that the spines of the snail keep off fouling critters, perhaps those that might drill through its thin and fragile shell. Still needs to be tested though…
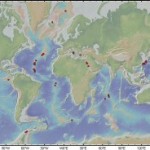
Biogeography Originally described from the Mariana Trough west of the Philippines, these snails populate many of the back-arc basins in the western Pacific, including the Manus, North Fiji, and Lau back-arc basins. Additionally, A. hessleri is reported from the Central Indian Ridge. Genetic data suggest there is 2 genetically distinct populations living hundreds of meters apart in the North Fiji Basin, the dominant genotype being most similar to populations from the Manus Basin (5). Populations from the Lau Basin are also genetically separated from those from the North Fiji Basin (1). Furthermore, populations from southwest Pacific back-arc basins are distinct from those from the Mariana Trough are distinct from all of the western Pacific back-arc basins (6). The Central Indian Ridge populations of Alviniconcha are distinct from all other populations (4, 13), though most closely related to populations from the Mariana Trough (4).

Further Reading
- Denis, F., D. Jollivet, and D. Moraga. 1993. Genetic separation of two allopatric populations of hydrothermal snails Alviniconcha spp. (Gastropoda) from two south western Pacific back-arc basins. Biochemical Systematics and Ecology 21:431-440.
- Desbruyères, D., A.-M. Alayse-Danet, S. Ohta, and Scientific Parties of BIOLAU and STARMER cruises. 1994. Deep-sea hydrothermal communities in Southwestern Pacific back-arc basins (the North Fiji and Lau Basins): composition, microdistribution and food web. Marine Geology 116:227-242.
- Hasegawa, K., K. Fujikura, and T. Okutani. 1997. Gastropod fauna associated with hydrothermal vents in the Mariana Back-Arc Basin: summary of the results of 1996 “Shinkai 6500” dives. JAMSTEC Journal of Deep Sea Research 13:69-83.
- Kojima, S., K. Fujikura, T. Okutani, and J. Hashimoto. 2003. Phylogenetic relationship of Alviniconcha gastropods from the Indian Ocean to those from the Pacific Ocean (Mollusca: Provannidae) revealed by nucleotide sequence of mitochondrial DNA. Venus (Japan Journal of Malacology) 63:65-68.
- Kojima, S., S. Ohta, T. Miura, Y. Fujiwara, and J. Hashimoto. 2000. Molecular phylogeny study of chemosynthetic-based communities in the Manus Basin. JAMSTEC Journal of Deep Sea Research 16:7-13.
- Kojima, S., R. Segawa, Y. Fijiwara, K. Fujikura, S. Ohta, and J. Hashimoto. 2001. Phylogeny of hydrothermal-vent-endemic gastropods Alviniconcha spp. from the Western Pacific revealed by mitochondrial DNA sequences. Biological Bulletin 200:298-304.
- Okutani, T., and S. Ohta. 1988. A new gastropod mollusk associated with hydrothermal vents in the Mariana Back-Arc Basin, Western Pacific. Venus (Japan Journal of Malacology) 47:1-9.
- Pranal, V., A. Fiala-Medioni, and J. Guezennec. 1997. Fatty acid characteristics in two symbiont-bearing mussels from deep-sea hydrothermal vents of the south-western Pacific. Journal of the Marine Biological Association of the UK 77:473-492.
- Stein, J. L., S. C. Cary, R. R. Hessler, S. Ohta, R. D. Vetter, J. J. Childress, and H. Felbeck. 1988. Chemoautotrophic symbiosis in a hydrothermal vent gastropod. Biological Bulletin 174:373-378.
- Suzuki, Y., S. Kojima, T. Sasaki, M. Suzuki, T. Utsumi, H. Watanabe, H. Urakawa, S. Tsuchida, T. Nunoura, H. Hirayama, K. Takai, K. H. Nealson, and K. Horikoshi. 2006. Host-Symbiont Relationships in Hydrothermal Vent Gastropods of the Genus Alviniconcha from the Southwest Pacific. Appl. Environ. Microbiol. 72:1388-1393.
- Suzuki, Y., T. Sasaki, M. Suzuki, Y. Nogi, T. Miwa, K. Takai, K. H. Nealson, and K. Horikoshi. 2005. Novel chemoautotrophic endosymbiosis between a member of the Epsilonproteobacteria and the hydrothermal-vent gastropod Alviniconcha aff. hessleri (Gastropoda: Provannidae) from the Indian Ocean. Applied and Environmental Microbiology 71:5440-5450.
- Urakawa, H., N. Dubilier, Y. Fujiwara, D. E. Cunningham, S. Kojima, and D. A. Stahl. 2005. Hydrothermal vent gastropods from the same family (Provannidae) harbour e- and g-proteobacterial endosymbionts. Environmental Microbiology 7:750-754.
- Van Dover, C. L., S. E. Humphris, D. Fornari, C. M. Cavanaugh, R. Collier, S. K. Goffredi, J. Hashimoto, M. D. Lilley, A. L. Reysenbach, T. M. Shank, K. L. Von Damm, A. Banta, R. M. Gallant, D. Götz, D. Green, J. Hall, T. L. Harmer, L. A. Hurtado, P. Johnson, Z. P. McKiness, C. Meredith, E. Olson, I. L. Pan, M. Turnipseed, Y. Won, C. R. Young III, and R. C. Vrijenhoek. 2001. Biogeography and ecological setting of Indian Ocean hydrothermal vents. Science 294:818-823.
- Warén, A., and P. Bouchet. 1993. New records, species, genera, and a new family of gastropods from hydrothermal vents and hydrocarbon seeps. Zoologica Scripta 22:1-90.
- Wittenberg, J. B., and J. L. Stein. 1995. Hemoglobin in the symbiont-harboring gill of the marine gastropod Alviniconcha hessleri. Biological Bulletin 188:5-7.





Hairy crabs, hairy snails, … what’s next?
Nice article, Kevin. Thanks! I hope you do more.
Great stuff! But I might be biased …
Hmm… I wonder what else is hairy in the deep sea
Are they operculate? How do they avoid predation from the crabs with those thin shells?
Yep, they have a thin operculum. I’m not sure how they keep from being predated, but a few observations from the field:
1) many of these snails are covered in white bacteria
2) when I crushed some snails incidentally, the crabs came pouring out of the woodwork toward the crushed snails.
So, it is unclear what the palatability of the snails are but it seems they were probably being eaten by the crabs. Now, the reason why this certain crab may be highly associated with the snail in the first place is because it might be grazing the the bacteria off the snails shell. Crabs are very opportunistic and if they sense an easy snack they might go for an injured snail. We don’t have much evidence to back up the claim, but just one of several things to consider.
Thanks for you question!